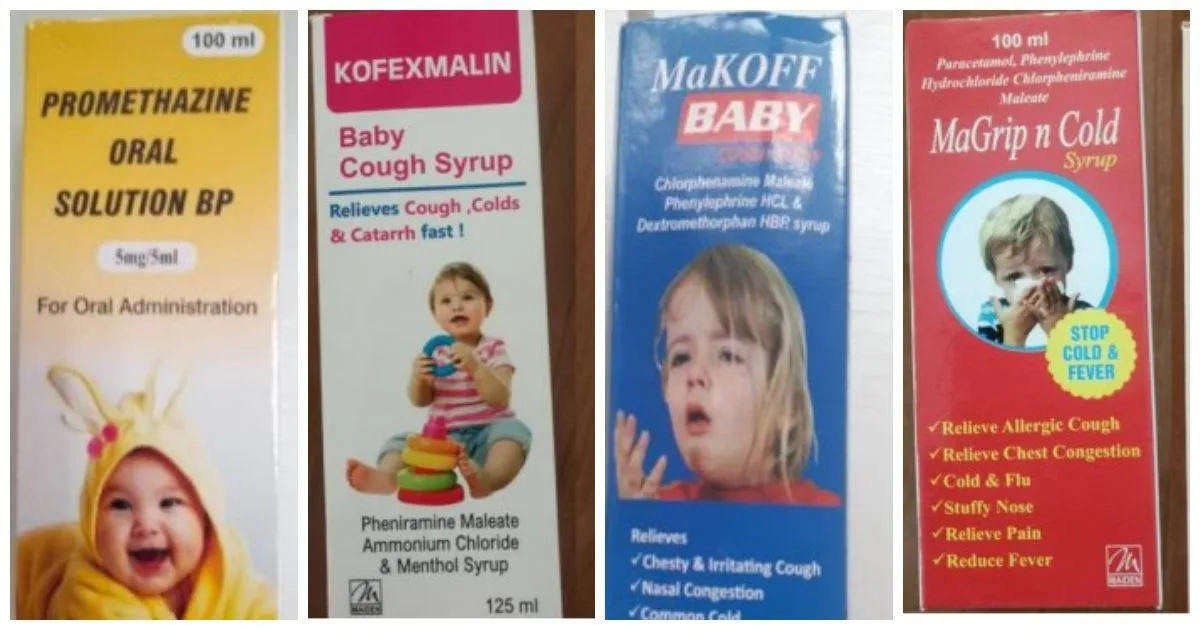
The World Health Organization (WHO) has given a global alert on four cough syrups made by the Delhi-based Maiden Pharmaceuticals in India that these drugs could lead to serious injury or death among children.
The alert was given after 66 children, who were administered the syrups, died in the Gambia. Medical investigations have revealed that the cough syrups can result in acute kidney injury in children less than five years of age.
Maiden Pharmaceuticals’ Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup, and Magrip N Cold Syrup are now suspended in the Gambia. The government is also running an urgent door-to-door recall of these cough syrups.
WHO said these syrups contain “unacceptable amounts of diethylene glycol and ethylene glycol as contaminants.” Laboratory analysis has also indicated traces of E.Coli in the medicine samples.
The world health body said these drugs could have reached other markets as well, through informal sources. It also suspects that the manufacturer, Maiden Pharmaceuticals, could have used these contaminated medicines in its other formulations distributed locally or exported. Hence the global alert on the products.
The Union Health ministry here responded to the issue by saying that the Drug Controller General of India has taken up the matter immediately after it was informed about it on September 29. It is investigating the matter in collaboration with the Haryana State Regulatory Authority, within whose jurisdiction comes the manufacturing unit of Maiden Pharmaceuticals.
Meanwhile, the Central Drugs Standard Control Organization (CDSCO) has sent “controlled samples of the same batch of the four drugs” to the Regional Drug Testing Lab in Chandigarh. CDSCO has also requested WHO for causal evidence linking these cough syrups to the deaths reported.
The Indian health ministry also said that the cough syrups in question are not sold in the local market and are exported to only Gambia. The statement from the ministry further added, “It is usual practice that the importing country tests these imported products on quality parameters and satisfies itself as to the quality of the products before the importing country decides to release such products for usage in the country.”
Meanwhile, speaking on the issue, Gambia health services director Mustapha Bittay has said that the country, as of now, does not have a medicine-testing laboratory and is dependent on labs abroad. Mr. Bittay has told the BBC that the Gambia is holding discussions with the World Bank to seek funding for a quality-control laboratory.
TNV view: India should urgently review the safety of all drugs that are exported but not sold in the country. We must take the onus of testing for safety when we export medicines to countries like the Gambia, which does not have the infrastructure for the same.